UK SPINE Proof of Concept Programme

Health impacts of biological aging impose a significant burden on individuals and on society [1]. Associated with increased frailty [2], age is also the primary risk factor for diseases such as cancer, cardiovascular disease, and neurodegeneration [3]. Age-associated multimorbidity further exacerbates the burden [4], and these multiple morbidities tend to occur in clusters [5, 6].
Descent into multimorbidity from onset of initial disease is accompanied by increased reliance on medical intervention, increased need for supportive care, and, often, reduced ability to participate productively in society [5, 7].
Focused on alleviating this burden, the UK SPINE brings together a network of experts and resources to discover drugs which modulate biological drivers of unsuccessful aging. Preventing progression to disease and multimorbidity is the collective goal.
Prevention: Inherently targeting multimorbidity
Lifestyle choices play a vital role in maintaining good health; nonetheless, effective medicines targeting biological aging could yield significant benefits: from the age of 45, the lifetime risk of non-communicable disease exceeds 90% for both men and women [8]. By centring prevention, drugs may coordinate with natural biological processes to collectively increase healthspan. Furthermore, prolonging successful aging to hold age-associated multimorbidities at bay will reduce polypharmacy, which carries its own risks [9].
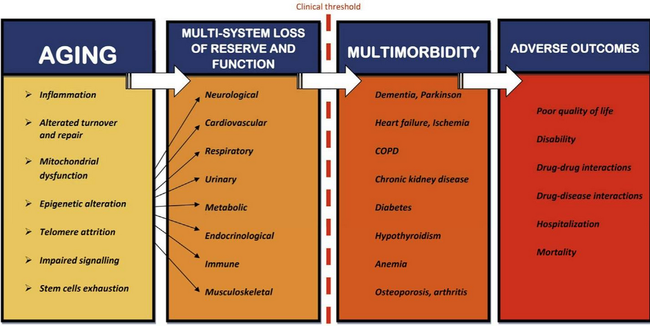
Figure and caption from [4]: “The age-related multisystem loss of reserve and function, rooted in the biological determinants of the aging process, is associated with increased susceptibility for chronic diseases, which becomes clinically evident as multimorbidity when a certain threshold of impairment has been a reached.”
Identifying and developing safe, effective interventions for aging is a formidable challenge. The biology of aging is not fully understood, and the impact of age on individuals is highly variable. While biological pathways of aging are embedded in complex, dynamic, multiplexed systems, scientists have described a set of key hallmarks of aging [9]. Numerous studies have identified biological pathways and targets by which progression may be modulated [10, 11], and characterization of biomarkers for assessing biological aging [12] is underway. New and repurposed drugs influencing those pathways are being investigated for efficacy and safety [13, 14], and ongoing research to identify additional pathways and targets will reveal leads for new drugs.
Drug discovery: Networks, not pipelines
The UK SPINE, supported by Research England (UKRI) via the Connecting Capability Fund (CCF), aims to establish an unconstrained flow of resources and expertise within a complex drug discovery network, toward accelerating the route to clinic for promising interventions.
Drug discovery is traditionally characterized as a pipeline, leading from basic research to the clinic. A linear path between singular start and end points belies a more complex reality: drug discovery involves coordination of distributed resources, alongside dynamic inputs from a broad range of contributors interspersed throughout the network.
The UK SPINE’s five founding hub institutions provide the framework for a comprehensive drug discovery network spanning basic biological science, target validation, hit identification, medicinal chemistry, preclinical validation, and clinical trials. Around these hubs, an extended network supporting efficient exchange of resources and expertise is being cultivated: participants include academia, research institutes, the NHS, patient groups, pharma, SMEs, charities, regulators, investors, entrepreneurs.
This collective of diverse contributors offers a unique opportunity to seed novel cross disciplinary interactions, establish new collaborations, and promote early engagement with downstream partners. By optimally connecting distributed knowledge and resources, the UK SPINE capitalizes on the networked nature of drug discovery, transcending the linear pipeline meme.
Knowledge exchange: Essential to drug discovery
Drug discovery relies on a coordinated exchange of resources within an underlying network, with each point of connection an interface to be navigated. Innovation relies on novel approaches bolstered by collaboration and unfettered access to both data and expertise. Timely and efficient access to reagents, equipment, and biological samples are also critical to rapid progress. Ultimately, transparent and efficient orchestration of expertise and resources will ensure that new drugs get to clinic with minimal delay.
Designed to bridge gaps, promote openness, and facilitate speedy access to and distribution of resources, the UK SPINE’s CCF-funded knowledge exchange (KE) efforts aim to optimize free flow of knowledge and resources within a coordinated network that spans the UK.
KE activity streams
The UK SPINE’s KE efforts to date focus on several fronts which influence fluidity in drug discovery:
- To speed administrative and contractual aspects of cross-institutional interactions, standardized agreements have been established to support a variety of collaborations and engagements.
- Early attention to the nascent policy and regulatory environment for aging-specific indications and primary outcomes in clinical trials is driving advances which support the new paradigm of prevention-focused interventions.
- Partnerships to nurture and facilitate translation are being developed with pharma, investors, government, patient groups, downstream research partners, funders, and entrepreneur-focused technology transfer programs.
Importantly, the UK SPINE is pushing the envelope on knowledge exchange as a driver of drug discovery, experimenting with novel approaches, promoting engagement between traditionally distant actors in the network, and identifying where and in what form KE innovation might be of value.
Proof of Concept Program: Gathering intelligence, crafting solutions
The UK SPINE Proof of Concept (PoC) Program offers a unique, real world view on challenges impeding progress in discovery of drugs for aging. Funds have been awarded to 15 research projects targeting hallmarks of aging and representing key stages of discovery. The UK SPINE works with project researchers to resolve KE challenges and to identify opportunities for new KE initiatives, from streamlining access to resources to establishing early engagement with downstream partners. The key objective is to translate intelligence gleaned from the PoC Program into assets for wider adoption by the network.
Divided into two streams, the PoC Program consists of Flagship and Bridge portfolios. Flagship portfolio projects involve coordination across multiple institutions; these ambitious projects have specific goals intended to significantly advance toward clinical solutions. Complementing Flagship projects, the Bridge portfolio consists of a diverse group of nimble, exploratory studies. Selected primarily to advance the scientific state of the art, Bridge projects range from elucidation of biological aging pathways for target identification to development of biomarkers for clinical trials and diagnostic applications.
Projects supported by the UK SPINE PoC Program are advancing the state of the art in drug discovery for healthy aging. These exciting projects are also a conduit for intelligence essential to optimizing and accelerating the discovery process. Contributing uniquely valuable insights, researchers participating in the PoC Program play a key role in enriching UK SPINE KE initiatives. In the coming months, we look forward to disseminating outcomes of these collaborative efforts toward accelerated drug discovery in support of healthier aging.
References
[1] World Health Organization. Global Health and Aging (2011). NIH Publication no. 11-7737. Available at: https://www.who.int/ageing/publications/global_health.pdf
[2] Clegg A, et al. Frailty in elderly people. Lancet 2013;381(9868):752-62
[3] Niccoli T, Partridge L. Ageing as a risk factor for disease. Current Biology 2012;22(17):R741-R752
[4] Fabbri E, et al. Aging and Multimorbidity: New Tasks, Priorities, and Frontiers for Integrated Gerontological and Clinical Research. Journal of the American Medical Directors Association 2015;16(8):640-647
[5] Academy of Medical Sciences. Multimorbidity: a priority for global health research: Full report (2018). Available at: https://acmedsci.ac.uk/file-download/82222577
[6] Zemedikun D, et al. Patterns of multimorbidity in middle-aged and older adults: An analysis of the UK biobank data. Mayo Clinic Proceedings 2018;93(7):857-866
[7] The Lancet. Making more of multimorbidity: An emerging priority. Lancet 2019;391(10131):1637
[8] Licher S, et al. Lifetime risk and multimorbidity of non-communicable diseases and disease-free life expectancy in the general population: A population-based cohort study. PLoS Medicine 2019; https://doi.org/10.1371/journal.pmed.1002741
[9] Wastesson JW, et al. An update on the clinical consequences of polypharmacy in older adults: a narrative review. Expert Opinion on Drug Safety 2018;17:12, 1185-1196
[10] López-Otín C, et al. The hallmarks of aging. Cell 2013;153(6):1192-1217
[11] Melzer D, et al. The genetics of human ageing. Nature Reviews Genetics 2020;21:88-101
[12] Aunan JR, et al. Molecular and biological hallmarks of ageing. British Journal of Surgery 2016;103(2):e29-e46
[13] Guerville F, et al. Revisiting the Hallmarks of Aging to Identify Markers of Biological Age. The Journal of Prevention of Alzheimer’s Disease 2020;7:56-64
[14] Justice JN, et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 2019;40:554–563
[15] Barzilai N, et al. Metformin as a tool to target aging. Cell Metabolism 2016;23(6):1060-1065